
Exploring Electrical Properties of Mesenchymal Stem Cells via Dielectrophoresis
By: Sierra Knowles Email: know7971@vandals.uidaho.edu
Home Town: Meridian, ID High School: Capital High School, 2018
Major: Chemical Engineering
Department: Chemical & Materials Engr
College: College of Engineering
Abstract
Stem cells play an important role in regenerative medicine because of their ability to proliferate into various cell types. Current work focuses on murine mesenchymal stem cells (MSCs) differentiating into tenogenically differentiated cells upon exposure to a growth factor TGFβ2. During the course of their differentiation on treatment, MSCs are observed to exhibit different phenotypical and genotypical changes accounting for their change in electrophysiological make-up, i.e., the capacitance and permittivity of both the cell membrane and its interior. In this study, murine MSCs are characterized using dielectrophoresis (DEP), a non-labeling, non-destructive electrokinetic technique using a crossover frequency method. DEP is known to be able to detect subtle changes within the cell. Studies were performed on both untreated and treated (treated with growth factors differentiating into tenogenically modified MSCs) murine MSCs; the treated MSCs were treated on a time scale of days 1, 3, and 7. The results showed that tenogenically modified MSCs differed significantly in their electrical properties and could be distinguished from the untreated MSCs as early as 3 days into treatment.
Introduction
Mesenchymal Stem Cells
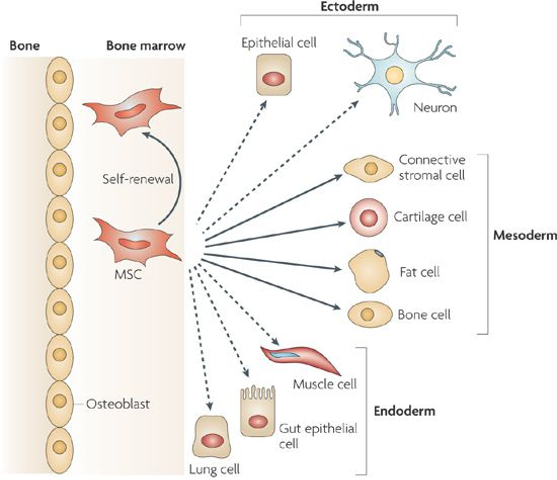
Mesenchymal stem cells are stem cells found in bone marrow & can differentiate into a variety of cell types, including bone cells, cartilage cells, muscle cells, and fat cells.
Dielectrophoresis (DEP)
Non-uniform electric field induces motion in polarizable particles, which is the termed as DEP. DEP is a label free and high throughput technique and is known to determine subtle morphological changes within biological entities. Motion of the dielectric particles towards high intensity field and low intensity fields are termed as n-dep and p-dep respectively. The frequency at which the net force acting is zero is termed as Dielectrophoretic crossover frequency.
GOAL: To determine the electrophysiological properties of murine mesenchymal stem cells using dielectrophoresis.
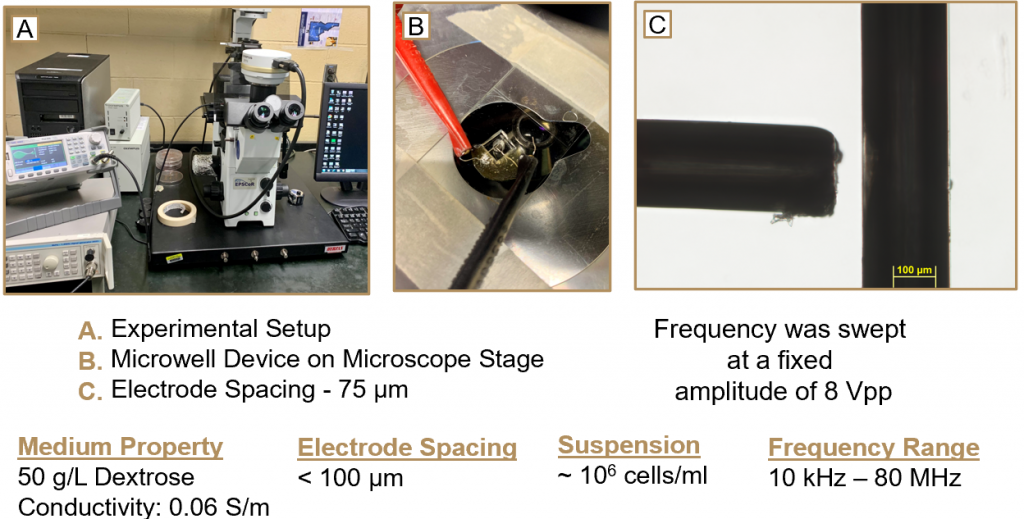
Experimental Setup
Experimental setup includes the fabrication of ‘microwell devices’ as shown in Figure B below. These devices contain thin platinum wires arranged in a ‘T’ shape as shown in Figure C below. Cells are transferred via pipette into the device, and an electrical current is ran through the wires, inducing dielectrophoresis.
Observations
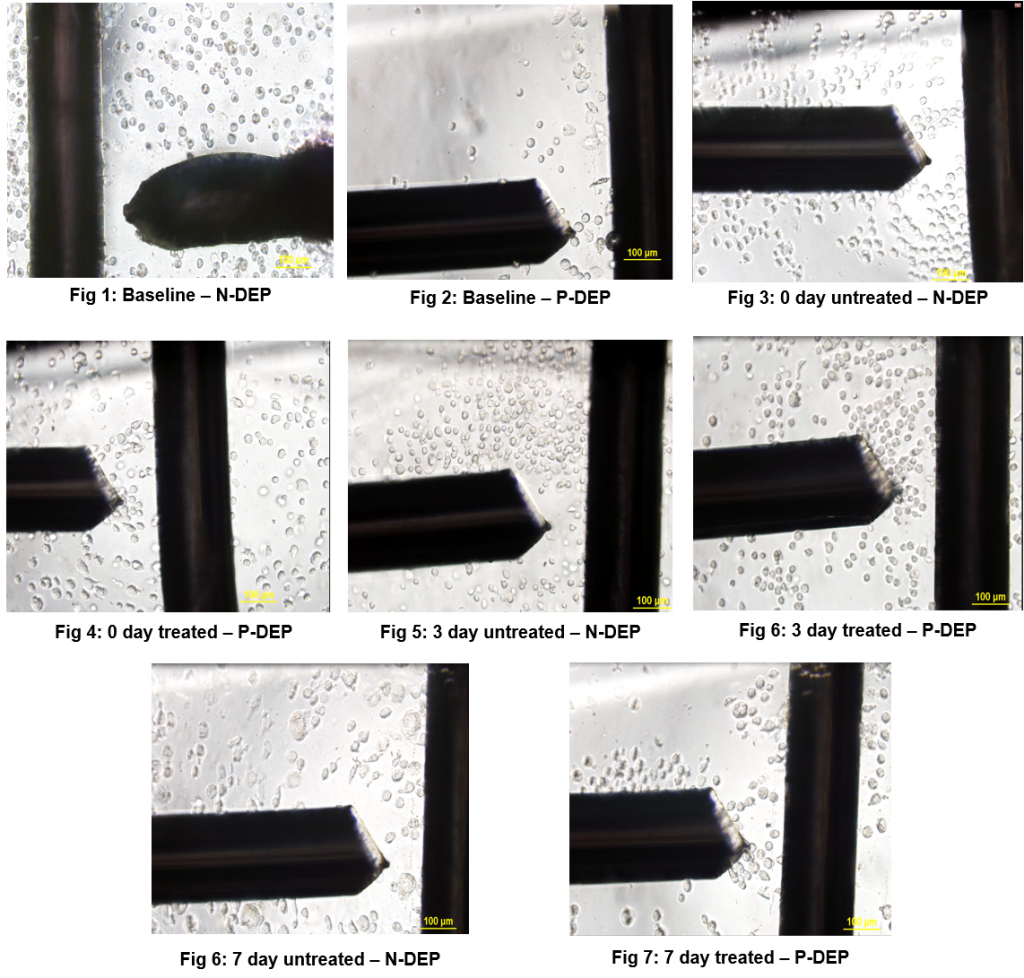
Dielectrophoretic experiments were performed by suspending 2µL of the prepared medium until crossover frequency was determined. Experiments were performed on 0, 3 and 7 day time points, and were ran 3 times each time point to check the consistency of the determined frequencies. Experiments were also performed on different devices to determine if variability of device effects results; It was noticed that the variation is ± 3%.
Results
Single – Shell Model
In order to obtain the properties of the cells (total membrane capacitance and permittivity), we modeled the stem cells as a single-shell model.
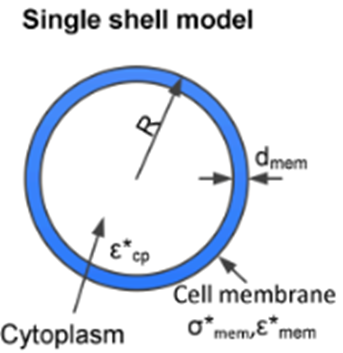
The main equations are as follows:
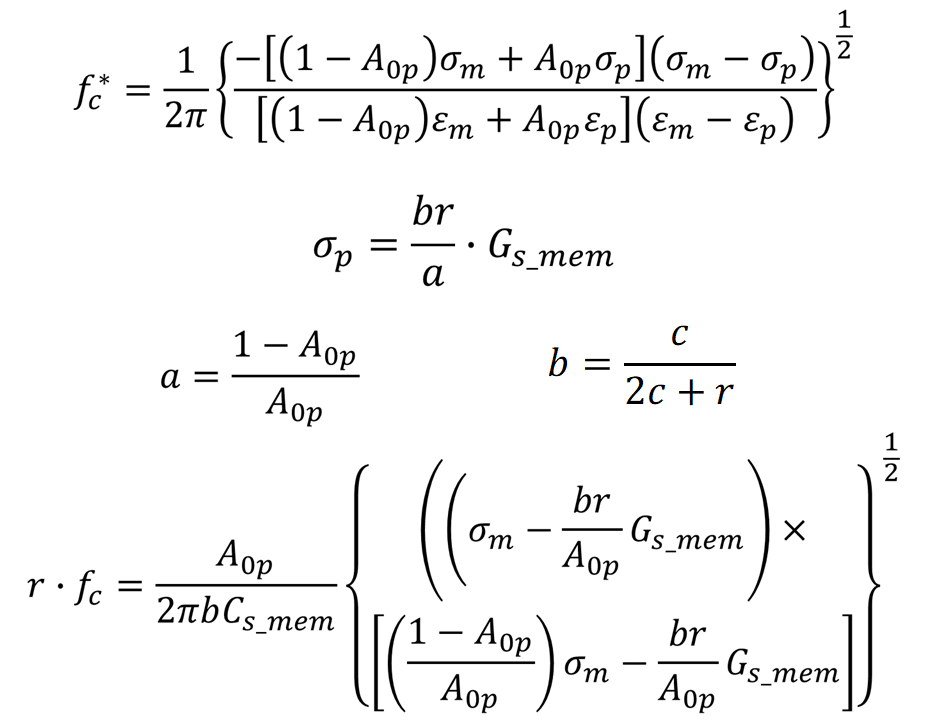
f_c = cross frequency obtained for a single shelled spheroids of radially-symmetric radius r rotating about a normal minor axis, c. ε and σ are the real permittivity and conductivity with the subscripts m and p representing medium and particle respectively. C and G are capacitance and conductance with the subscript s_mem denoting per unit area of particle. A_0p is given as 0.236.
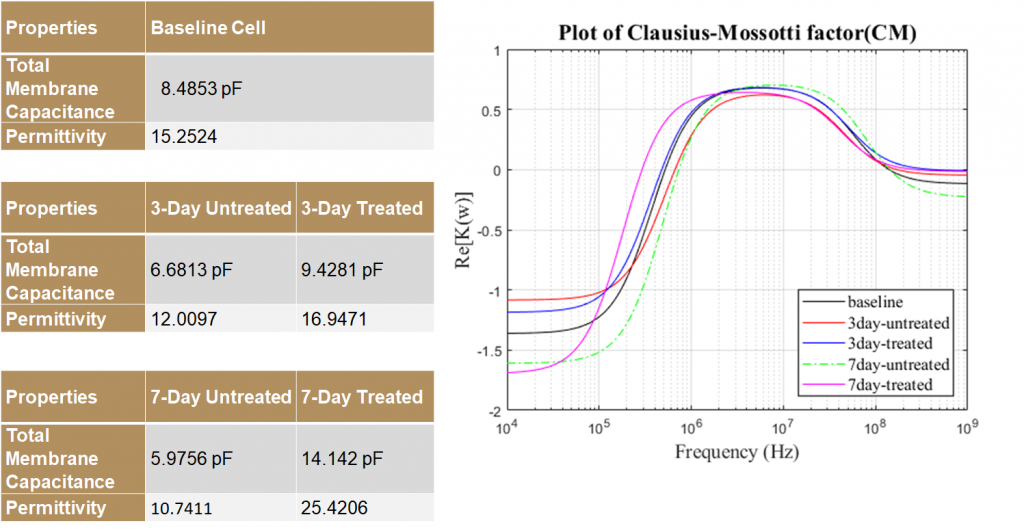
Properties and Crossover Frequencies
Discussion and Conclusions
The dielectric properties of MSCs vary significantly for different groups. Using this difference in properties, tenogenically differentiated cells can be sorted from the untreated controls as early as 3 days of treatment. TGFβ2, which is used to initiate the differentiation, affects cell dielectric properties significantly. The 0 day treated and untreated MSC’s displayed aberrance and aren’t significantly different from each other and separation of these may not be effective. This indicates that TGFβ2 –induced tenogenesis may require at least 24 hours to change the dielectric properties.
Future Work
Future work includes the development and modelling of a microfluidic sorting platform to separate stem cells of different time points using dielectric properties. The modelling of a microfluidic sorting platform will use COMSOL to optimize sensitivity and specificity. Further experiments will be done to test the validation and optimization of the sorting platform for different treatment groups.
References
• Adams, T. N. G., Jiang, A. Y. L., Vyas, P. D. & Flanagan, L. A. Separation of neural stem cells by whole cell membrane capacitance using dielectrophoresis. Methods 133, 91–103 (2018).
• Pethig, R., Menachery, A., Pells, S. & De Sousa, P. Dielectrophoresis: A review of applications for stem cell research. Journal of Biomedicine and Biotechnology 2010, (2010).
• Zhu, B. & Murthy, S. K. Stem cell separation technologies. Current Opinion in Chemical Engineering 2, 3–7 (2013).
Products Produced:
| Type: | Title: | Date Published/Presented: | DOI: |
|---|---|---|---|
| Poster | Electrophysiological Characterization of Mesenchymal Stem Cells via Dielectrophoresis | November 22, 2019 |
Additional Project Information:
Year in College Project Started: Sophomore
Faculty Advisor: Soumya Srivastava
Faculty Advisor Email: srivastavask@uidaho.edu
Faculty Advisor Website: https://www.uidaho.edu/engr/departments/cme/our-people/faculty/soumya-srivastava
Funding Source: This project was funded by the John F. Keegan Fellowship (to SKT). The travel to present this poster at Oregon State University Bioengineering Symposium 2019, Corvallis is supported by Office of Undergraduate Research Travel Grant, University of Idaho.
External Link to Project Information:
Project Location: Moscow, ID

