Developing microfluidic platform for electrokinetics characterization: C. Necator and Borrelia burgdorferi
By: Courtney Molvig Email: molv6350@vandals.uidaho.edu
Home Town: Nampa, Idaho High School: Nampa Senior High School, 2015
Major: Chemical Engineering, Chemistry-General Opt
Department: Chemical & Materials Engr, Chemistry
College: College of Engineering, College of Science
Abstract:
Cupriavidus necator, a Gram-negative soil bacterium, will be characterized using AC-based dielectrophoretic microwells. A crossover frequency will be found for the bacterium in buffer as well as the bacterium in different metal solutions and different concentrations. Once the crossover frequency has been observed and the process has been repeated to ensure repeatable values, the bacterium will be observed using an ultraviolet-visible spectrophotometer. Using the spectrophotometer will indicate whether the bacterium has absorbed the metal in the solutions. The amount of metal being absorbed by the bacterium can also be observed. Once the bacterium has been added to the metal solutions with a known concentration, an allotted amount of time will go by to allow the bacterium to absorb the metal solutions. A supernate will then be taken from the solution and observed using the spectrophotometer. The concentration of the solution can be found by using Beer’s Law to concert the absorbance to the concentration. This will determine if and how much the bacterium absorbed the metal from the solution. A crossover frequency for Borrelia burgdorferi, a causative agent of Lyme’s disease, will also be found using AC-based dielectrophoretic microwells.
Introduction:
Cupriavidus necator is a Gram-negative soil bacterium. C. necator is known for its ability to degrade various chemically-related pollutants. Studying this bacterium will allow for a better understanding of the mechanism used to degrade the chemically-related as well as a possible approach to help decrease the amount of pollutants found throughout the world. Borrelia burgdorferi is a causative agent of Lyme’s disease. Characterizing this bacterial species is important for creating a device capable of detecting whether a person has contracted Lyme’s disease.
Project Design:
Setting up for a microwell experiment:
The correct size of beaker (depending on the amount of polydimethylsiloxane (PDMS) that is being created) needs to be obtained. The required amount of silica elastomer and curing agent (a ratio of 10:1) needs to be weighed out. Once the amounts have been weighed, the solution must be stirred rapidly with a spatula for about a minute. The mixture is then vacuumed until the bubbles have been removed and the mixture is clear. After vacuuming, the solution is poured into a petri dish and placed in the oven for an hour at 70 ℃ . Once the time is up, the PDMS can be taken out of the oven and cut it up into small pieces. A hole, about 2 mm in size, can be punctured into the PDMS. After puncturing a hole and cleaning the PDMS and a glass
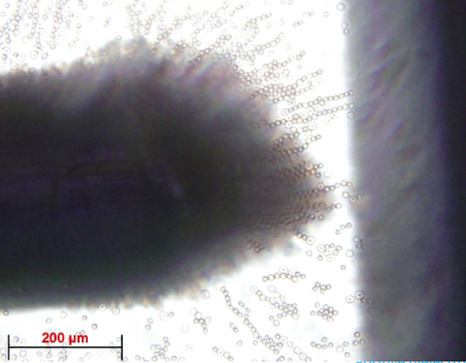
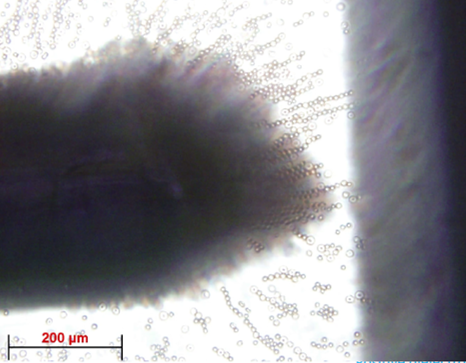
slide with tape, they can be plasma cleaned. Once plasma cleaning is done, the PDMS is inverted and place on the microscope slide. Once the PDMS is secured onto the microscope slide, platinum electrodes are added at a 90° angle and secure the ends with epoxy. The electrodes must be between 25 and 100 μm apart.
While the solution is in the oven, the buffer can be made. The buffer used for basic characterizations of c. necator and Borrelia burgdorferi is created by using 1.25 g of dextrose dissolved in 25 mL of deionized water. Once the solution is made, the pH and conductivity meters are used to ensure that the values fall within a given range. The buffer should have a pH close to 7 and the conductivity cannot be less than 0.055 S/m. If the conductivity is less than what is required, PBS can be added to increase it. Different concentrations of metal solutions can be prepared and stored in advance, but the buffer should be made fresh or if using a solution prepared prior the pH and conductivity must be measured to ensure that the buffer is still usable.
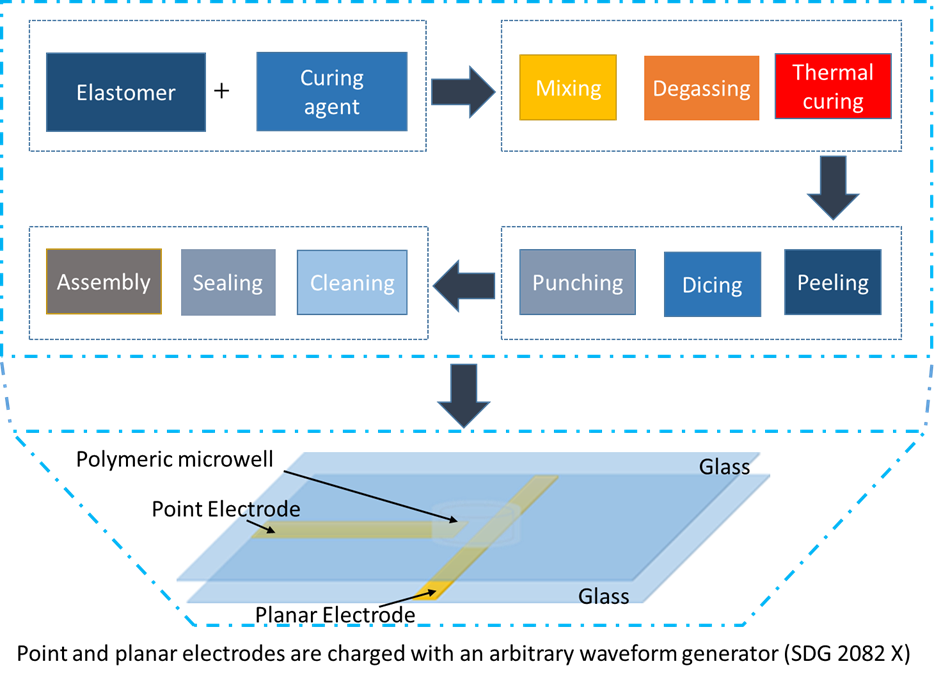
Dielectrophoresis-microwell experiments:
Borrelia burgdorferi cells that are put in buffer are added to the microwell device by using a micropipette and putting some of the solution into the hole that was punctured on the device. A non-uniform electric field is then applied through platinum electrodes of the microwell device. The frequency and amplitude of the voltage is varied until a crossover frequency is found.
Cupriavidus necator must be added into a container that contains buffer using aseptic technique. This solution is left to sit for twenty-four hours. At the twenty-four hour mark, about three milliliters of the solution is taken from the container and added to a new container with buffer. This solution is left to sit for twelve hours. Once the solution has sat for twelve hours, the experiment can begin. One and a half milliliters of cells and half a milliliter of ABS is added to a centrifuge container and centrifuged for fifteen minutes at 10,000 rotations per minute (RPM). One milliliter of the solution is then taken out while being sure to not disrupt the pellet and one milliliter of fresh ABS is added to the container and mixed well. The solution is centrifuged again for the same amount of time and RPM. The next step depends whether you are using the buffer or metal solutions. If you are using buffer to characterize the bacterium, you take out a specific amount of solution and add that amount of buffer to the solution and the process continues the same as it does for Borrelia burgdorferi cells. However, if you are using a metal solution you then take out a certain amount of solution and add that amount of metal solution to the container. You let the solution sit in the refrigerator for one hour before adding the solution to the microwell device and finding the crossover frequency.
UV/Vis:
Once a metal solution is added to the Cupriavidus necator solution and is allowed to sit for an hour. The supernate of the solution is taken and added to a cuvette. An absorbance is than taken from the device and can be converted to concentration using Beer’s Law. This information will identify the amount of metal the bacterium absorbed.
With collaboration from my peers in MESA lab, I will help characterize Cupriavidus necator using AC-based dielectrophoretic microwells. With the information of the crossover frequency and the amount of metals the bacterium has absorbed will give a possible approach on decreasing chemical pollutants. Characterization of Borrelia burgdorferiusing AC-based dielectrophoretic microwells will also be performed. Once the dielectric properties of Borrelia are obtained, we will use the data found to modeling and running simulations on COMSOL. The will allow us to find a suitable design for the device, as well as the ideal operating voltage conditions. Once a suitable design for the device has been found, the device
will be fabricated and tested.
Literature/References:
1. Herman, Vos, Koen, Anneke, Jongh, D., Bartelt, . . . Jacob. (1993, October 01). Different Genospecies of Borrelia burgdorferi Are Associated with Distinct Clinical Manifestations of Lyme Borreliosis. Retrieved from https://academic.oup.com/cid/article-abstract/17/4/708/549138
2. Kang, C., Hayes, R., Sanchez, E. J., Webb, B. N., Li, Q., Hooper, T., . . . Xun, L. (2012, January). Furfural reduction mechanism of a zinc-dependent alcohol dehydrogenase from Cupriavidus necator JMP134. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3551575/?tool=pmcentrez
3. Lane, R. S., & Pascocello, J. A. (1989, October 01). Antigenic characteristics of Borrelia burgdorferi isolates from ixodid ticks in California. Retrieved from https://jcm.asm.org/content/27/10/2344.short
4. Lerch, T. Z., Dignac, M., Barriuso, E., & Mariotti, A. (2011, October). Effect of glucose on the fatty acid composition of Cupriavidus necator JMP134 during 2,4-dichlorophenoxyacetic acid degradation: Implications for lipid-based stable isotope probing methods. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21856833
5. Lerch, T. Z., Chenu, C., Dignac, M. F., Barriuso, E., & Mariotti, A. (2017, May 23). Biofilm vs. Planktonic Lifestyle: Consequences for Pesticide 2,4-D Metabolism by Cupriavidus necator JMP134. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5440565/?tool=pmcentrez
6. Li, Q., Metthew Lam, L. K., & Xun, L. (2011, November). Cupriavidus necator JMP134 rapidly reduces furfural with a Zn-dependent alcohol dehydrogenase. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21526390
7. Ma, Y., Seiler, K. P., Eichwald, E. J., Weis, J. H., Teuscher, C., & Weis, J. J. (1998, January 01). Distinct Characteristics of Resistance toBorrelia burgdorferi-Induced Arthritis in C57BL/6N Mice. Retrieved from https://iai.asm.org/content/66/1/161.short
8. Meriläinen, L., Herranen, A., Schwarzbach, A., & Gilbert, L. (2015, March). Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4339653/
Products Produced:
| Type: | Title: | Date Published/Presented: | DOI: |
|---|---|---|---|
| Poster |
Additional Project Information:
Year in College Project Started: Senior
Faculty Advisor: Soumya Srivastava
Faculty Advisor Email: srivastavask@uidaho.edu
Faculty Advisor Website: https://sites.google.com/view/mesalab
Funding Source: The Office of Undergraduate Research
External Link to Project Information:
Project Location: Moscow, Idaho